1) How do you calculate from injected test to testosterone blood levels? I understand this equation:
Sigma[ initialInjectionAmount * Exp[ Ln[1/2] (dayOfCycle-dayInjected)/halflife]
(can you tell that I'm a mathematica freak? btw, I find an equation manipulator such as mathematica is easier to use than a spreadsheet like Excel for this kind of model) This is just a summation of decreasing exponentials. Is the conversion from injected test to blood test levels a simple multiplication by a constant? If so, what constant? And, is the constant a function of the type of the type of aas?
This is based on the idea that when you inject and esterified AAS, it essentially have two half lives: The depot as an inactive, esterified compound (long half life) and the active compound in the blood once the ester has been removed. The latter is so small and insignificant (2hrs) that it is not taken into account.
2) Do we know that the pharmokinetics of the absorption of the estrified testosterone is such that the rate limiting step is a pure diffusion process (thereby explaining the simple decreasing exponentials?) I see this is probably true, once the estrified testosterone is converted to test and enters the body, since almost all medications have an associated half-life.
However, I have read that while the estrified test resides in oil, hydrolysis is not possible, and therefore the oil acts as a storage depot of the test. As a result, the curves may need to be amended to allow for a time delay of the test's entrance into the body. Now, it could be that this is, in fact, the rate limiting step, and that this process is purely concentration dependent, and therefore is a pure decreasing exponential, however, I just want to be sure of this.
There are indeed other factors that influence diffusion and de-esterification.. The main factors are volume of benzyl alcohol used and total AAS concentration. Secondary factors are size of injection and location.
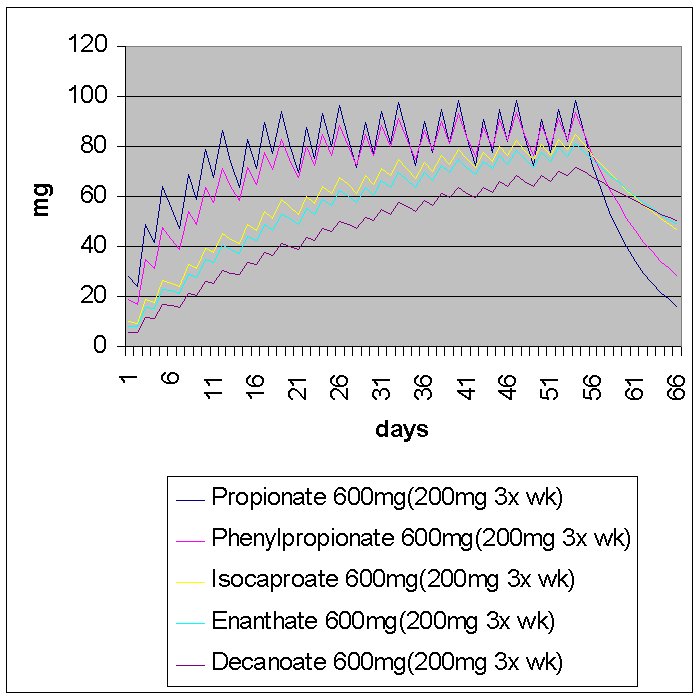
It is true that esterified testosterone (or any AAS) cannot be de-esterified in the oil. It takes a water molecule to break the ester linkage between the AAS and the carboxylic acid in which it is attached to. In addition to injection concnetration, it's certianly reasonable to think that some AAS and their esters can have greater affinity to estarase enzyme.. However, literature suggests this does not result in any measureable difference in de-esterification. So, the graphs represent blood androgen levels in as pure exponential decay models. Only the "first" half life, the rate at which the de-esterification proceeds is taken into consideration. I consider the other factors such as injection volume to contribute only marginally to the blood levels.
3) Theories are all well and good, but as you know, many appealing theories have been proven wrong. Has this model been corroborated with actual measurements of blood test levels? Is it always a pure decreasing exponential? Do test levels always peak immediately after the injection?
There have been graphs posted that were based on actual blood tests.. One in particular is that of testosterone enanthate. It was very indicative of the "theoretical" graphs I have made just using exponential decay. Some other actual blood graphs show deca peaking out on day 4-5.. This is just one of those things that cannot be taken into consideration. The theoretical graphs assume the AAS begins exponential decay immediately after injection. The 'actual' graphs that showed deca peaking on the fifth day also showed a pretty high amount of deca after the first 24 hrs.. Point being, all injectables peak early. In the case with the deca, there was little difference between the blood levels after day 1-2 and 4-5, meaning, as predicted by the "theoretical graphs," deca is de-esterified so slowly as a result of long ester that the amount release between days deviates very small compared to a faster injectable.. All of this follows amazingly easy from the simple mathematical model
4) Is it possible to extend this model to the synthetic aas's, i.e. to deca, winny, eq, etc. It could be that the reason that stacks are more effective is simply because there is a higher concentration of testosterone like molecules in the body. None of this mucking about with various receptor types, receptor binding strengths, class I&II steroids, etc. etc. Of course, these phenomena may exist, but they may be 2nd order effects, and that the most important thing is simply blood concentration of testosterone-like molecules.
The graphs apply to all types of esterified AAS. The graph is very general and it's hard to say we can slap it on any AAS, but from actual blood test, it apears that all esterified AAS follow this type of behavior. Non-esterified comounds like dbol and winstol do not follow such a graph. Their graph would be much more difficult to do since it has one real exponential half life.. And much more goes into it especially when the compounds are injected.. There is also a lot of debate on the half life of winstrol.. A 17aa group most definetly increases the half life but there isn't a lot of data on it.
5) Would you argue that an aas that results in large gains (eg test, ad50, dbol)) vs. one that results in "quality" gains (winny, eq, primo, etc.), is mostly (to 1st order) a function of estrification rate? I.e. winny, eq, and primo do not aromatize well, so therefore less estrogen, so therefore less water retention, so therefore more "quality" gains, vs. test, dbol, ad50, which aromatize easily, so therefore, more estrogen, so therefore more water retention, so therefore "lower quality "gains?
Absolutely. Compounds like trenbolone and deca are alleged to have strictly "AR effects" or effects directly through androgen receptor agonization. Comounds like dbol and andadrol are not good AR binders and cause the most water retention. Their mode of action is said to be largly "outside" of the AR. Keep in mind that documentaion of ANABOLISM from AAS that does not occur through the AR has never been demonstrated to exist. That's not to say that it doesnt.. I my opinion, AR agonization results in at least 90% of muscle growth.
6) Would you argue that doing a cycle stacked with orals at the beginning is exactly the same as front loading pure test?
No. Loading injectables means getting "AR binding AAS' up to speed. I think taking dbol in the beginning of a cycle is inferior to loading injectables.. As I said, it's my opinion that most of the growth occurs through the AR.. ANd loading those compounds is VER beneficail. Loading by taking dbol in the beginning just gives rise to a superficial bloated feeling that no doubt increases strenghth, but is inferior to loading of injectables.
7) Would you argue that an ideal cycle would tailor the halflives and injection times such that the overall blood testosterone levels approximate a step function up at the beginning of the cycle, and a step function down at the end? Or would an ideal cycle include tapering, to allow other systems in the body to accomodate the new substances?
Since your post, I have been calculating step function up and down cycles. You could achieve the former with front loading a long acting test such as enanthate (say 4 injections the 1st 2 days), inject the enanthate at a constant frequency (say, twice a week), and switch four weeks before the end of the cycle to a shorter acting test, such as propionate, injected more frequently (say eod). After the last propionate injection, immediately begin treatment of inhibition and cortisol levels. No wasted time waiting for injected test levels to slowly increase and equilibrate to some steady state level, at whichone may start seeing gains. And no wasted time waiting for test levels to decrease so that one may begin post cycle treatment.
That is exaclty why I advocate switching to shorter esters to end a cycle... I think the ideal graph of a cycle would look like a rectangle.. That is, it would be at it's highest blood levels for the entire cycle, from beginning to end.. ANd then it would END so that clomid therapy can begin. traditional cycles are pyramids, even if they inject the same amount throughout the cycle.. they are still pyramids.
8) Finally, what is the chemical formula for test? The esters, I understand, i.e. OOC-R, however, to calculate molecular weights, I need to know the formula for test.
I think the molecuar weight for test is 284.. But let me check on that.
Andy


 Please Scroll Down to See Forums Below
Please Scroll Down to See Forums Below