R
Ross
Guest
Tricking The HPTA
Imagine being able to run a 10 week cycle of testosterone with virtually NO suppression of endogenous testosterone production...
Several studies confirm that this very well MAY BE POSSIBLE! Simply by suppressing PROLACTIN.
Prolactin is a NASTY hormone: The major effect of increased prolactin is a decrease in normal levels of sex hormones — estrogen in women and testosterone in men.
Several studies have confirmed that by SUPRESSING PROLACTIN, you can TRICK the HPTA into thinking it is NOT ON CYCLE! You can DE-SENSITIZE the HPTA! When Prolactin was drastically reduced in the body, the HPTA remained relatively unaffected by TESTOSTERONE ADMINISTRATION! These results demonstrate that subnormal levels of PROLACTIN reduce the sensitivity of the hypothalamic-pituitary system to feedback inhibition by Testosterone.(Role of prolactin in the regulation of sensitivity of the hypothalamic-pituitary )
Furthermore, studies ALSO demonstrate that having HIGHER PROLACTIN levels while on cycle results in an INCREASED HPTA sensitivity, meaning FASTER SHUTDOWN OF THE HPTA! High levels of PROLACTIN appear to sensitize the hypothalamic-pituitary axis to the negative feedback effects of gonadal steroids. (Increased sensitivity to the negative feedback effects of testosterone induced by hyperprolactinemia in the adult male rat--McNeilly AS, Sharpe RM, Fraser HM.)
This all makes tremendous sense, as DECA and TRENBOLONE are the MOST suppressive AAS, and they bind AVIDLY to the progesterone receptor, drastically increasing PROLACTIN! This is why the HPTA shuts down! AND QUICKLY, as we can see from the graph below.
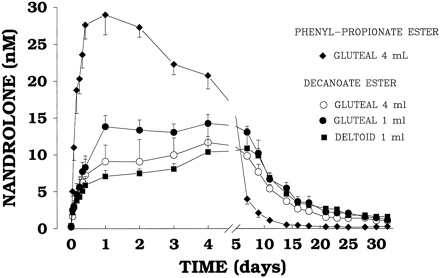
You can see from the chart above that a single 100mg injection of Deca caused a total (100%) reduction of natural testosterone levels, and it took approximately a full month to return those testosterone levels just to baseline! Just one shot!
In my experience with using Cabergoline(which suppresses prolactin), I can only confirm that this may have GENUINE REAL-WORLD APPLICABILITY. Not only does my libido go through the ROOF, my testicles mysteriously seem LARGER while on cycle. I also seem to experience VERY powerful orgasms and a MUCH shorter recovery time in between sexual sessions. I have personally never used DECA or TREN, but many of my friends and colleagues who HAVE, reported HUGE increases in libido and testicular mass while using either compound with CABERGOLINE! Increases in libido on DECA and TREN? Yep...
Let's take a closer look at some of these studies!
Role of prolactin in the regulation of sensitivity of the hypothalamic-pituitary system to steroid feedback.
Bartke A,
Matt KS,
Steger RW,
Clayton RN,
Chandrashekar V,
Smith MS.
Department of Physiology, Southern Illinois University, School of Medicine, Carbondale 62901.
During sexual maturation, pituitary gonadotropins stimulate the gonads to produce increasing amounts of biologically active steroids and yet gonadotropin release does not become suppressed until concentrations of sex hormones, LH and FSH, in peripheral circulation stabilizes at a higher adult level. There is a substantial amount of evidence that in many mammals, this transition from prepubertal to adult level of activity of the pituitary-gonadal axis is associated with a reduction in the sensitivity of the hypothalamic-adenohypophyseal system to negative feedback of gonadal steroids. In the female, these changes are accompanied by the appearance of positive estrogen feedback on gonadotropin release. In seasonal breeders, annual transitions between the periods of gonadal activity and quiescence are associated with corresponding shifts in the sensitivity to steroid feedback. Peripheral levels of pituitary prolactin (PRL) typically increase during sexual maturation and exhibit large seasonal fluctuations in response to changes in photoperiod and ambient temperature. We propose that PRL is one of the factors which regulate the sensitivity of gonadotropin release to gonadal steroid feedback. In hyperprolactinemic women, responsiveness to negative estrogen feedback increases, while LH response to positive estrogen feedback is reduced or absent. In hyperprolactinemic men, both LH and testosterone levels are reduced, implying increased sensitivity of LH release to negative testosterone feedback. In the male rat, both physiological amounts of PRL and experimentally-induced hyperprolactinemia increase the ability of exogenous testosterone to suppress LH and FSH release. Different regulatory mechanisms appear to operate in the seasonally breeding male golden hamster, in which short photoperiod causes concomitant suppression of PRL, LH, FSH and testosterone release. In this species, pharmacologic suppression of PRL release leads to increased responsiveness of plasma gonadotropin levels to negative feedback effects of testosterone, while PRL-secreting ectopic pituitary transplants exert an opposite effect. We have examined some of the suspected mechanisms of PRL modulation of testosterone feedback in male golden hamsters. In immature animals, the amount of cytoplasmic androgen receptors in the anterior pituitary was decreased by mild hyperprolactinemia and increased by treatment with bromocriptine, an inhibitor of PRL release. Bromocriptine increased pituitary androgen binding also in adult hamsters. These findings would imply that PRL modulates the responsiveness to negative steroid feedback at the pituitary level.(ABSTRACT TRUNCATED AT 400 WORDS)
PMID: 3324676 [PubMed - indexed for MEDLINE]
Prolactin modulates the gonadotropin response to the negative feedback effect of testosterone in immature male rats.
Chandrashekar V,
Bartke A,
Sellers K.
The effects of hyperprolactinemia (hyperPRL) and hypoprolactinemia (hypoPRL) on pituitary gonadotropin secretion and the feedback sensitivity to testosterone (T) were evaluated in immature male rats. At 34 days of age, rats were divided into three groups: group 1, controls, injected with oil; group 2, treated with bromocriptine mesylate (CB-154; 250 micrograms in oil/rat X day); and group 3, subjected to the transplantation of one pituitary from an adult female rat under the kidney capsule and treated with oil. The treatments were continued for 14 days. On day 8, each of these groups were further divided into three subgroups: intact, castrated, and castrated plus T treated. T treatment consisted of injection of T propionate (TP; 50 micrograms in oil/rat) on alternate days starting immediately after castration. Blood samples were obtained by cardiac puncture throughout the study. Plasma PRL levels were significantly reduced by CB-154 treatment and significantly increased by the pituitary graft (P less than 0.001). In intact immature male rats, hyper- or hypoPRL did not affect plasma LH levels, whereas hyperPRL reduced (P less than 0.01) plasma FSH concentrations. The postcastration increase in circulating LH levels was significantly increased (P less than 0.001) in rats treated with CB-154 24 h after castration. Moreover, the suppressive effects of TP on plasma LH and FSH levels were significantly (P less than 0.05) attenuated in hypoPRL rats. In pituitary-grafted rats, effects of castration and TP replacement on plasma LH levels did not differ from those in control rats. These results demonstrate that subnormal levels of PRL reduce the sensitivity of the hypothalamic-pituitary system to feedback inhibition by T. In contrast to previous findings in the adult rats, acute hyperPRL in immature male rats did not affect the negative feedback action of T on gonadotropin secretion.
PMID: 3100279 [PubMed - indexed for MEDLINE]
1: Biol Reprod. 1987 Feb;36(1):138-47. Links
Effects of hyperprolactinemia on the control of luteinizing hormone and follicle-stimulating hormone secretion in the male rat.
Smith MS,
Bartke A.
Experiments were conducted to determine the effects of acute hyperprolactinemia (hyperPRL) on the control of luteinizing hormone and follicle-stimulating hormone secretion in male rats. Exposure to elevated levels of prolactin from the time of castration (1 mg ovine prolactin 2 X daily) greatly attenuated the post-castration rise in LH observed 3 days after castration. By 7 days after castration, LH concentrations in the prolactin-treated animals approached the levels observed in control animals. HyperPRL had no effect on the postcastration rise in FSH. Pituitary responsiveness to gonadotropin hormone-releasing hormone (GnRH), as assessed by LH responses to an i.v. bolus of 25 ng GnRH, was only minimally effected by hperPRL at 3 and 7 days postcastration. LH responses were similar at all time points after GnRH in control and prolactin-treated animals, except for the peak LH responses, which were significantly smaller in the prolactin-treated animals. The effects of hyperPRL were examined further by exposing hemipituitaries in vitro from male rats to 6-min pulses of GnRH (5 ng/ml) every 30 min for 4 h. HyperPRL had no effect on basal LH release in vitro, on GnRH-stimulated LH release, or on pituitary LH concentrations in hemipituitaries from animals that were intact, 3 days postcastration, or 7 days postcastration. However, net GnRH-stimulated release of FSH was significantly higher by pituitaries from hyperprolactinemic, castrated males. To assess indirectly the effects of hyperPRL on GnRH release, males were subjected to electrical stimulation of the arcuate nucleus/median eminence (ARC/ME) 3 days postcastration. The presence of elevated levels of prolactin not only suppressed basal LH secretion but reduced the LH responses to electrical stimulation by 50% when compared to the LH responses in control castrated males. These results suggest that acute hyperPRL suppresses LH secretion but not FSH secretion. Although pituitary responsiveness is somewhat attenuated in hyperprolactinemic males, as assessed in vivo, it is normal when pituitaries are exposed to adequate amounts of GnRH in vitro. Thus, the effects of hyperPRL on pituitary responsiveness appear to be minimal, especially if the pituitary is exposed to an adequate GnRH stimulus. The suppression of basal LH secretion in vivo most likely reflects inadequate endogenous GnRH secretion. The greatly reduced LH responses after electrical stimulation in hyperprolactinemic males exposed to prolactin suggest further that hyperPRL suppresses GnRH secretion.
PMID: 3105612 [PubMed - indexed for MEDLINE]
1: Endocrinology. 1984 Oct;115(4):1506-10. Links
Does prolactin modify testosterone feedback in the hamster? Pituitary grafts alter the ability of testosterone to suppress luteinizing hormone and follicle-stimulating hormone release in castrated male hamsters.
Bartke A,
Matt KS,
Siler-Khodr TM,
Soares MJ,
Talamantes F,
Goldman BD,
Hogan MP,
Hebert A.
Adult male golden hamsters maintained in a long photoperiod (14 h of light and 10 h of darkness) or in a short photoperiod (5 h of light and 19 h of darkness for 7 weeks) were castrated and either given one anterior pituitary transplant under the kidney capsule or sham-operated. Additional animals were castrated and grafted or sham-grafted at the time of transfer to the short photoperiod. Starting 2 weeks after castration, all animals were injected three times a week with 20 micrograms testosterone propionate (TP). After 3 weeks, the dose of TP was increased to 80 micrograms and, after an additional 2 weeks, to 320 micrograms per injection. Blood samples were collected 2 weeks after castration and 1 day after the last injection of 20, 80, and 320 micrograms TP. Short photoperiod reduced and pituitary grafts increased plasma PRL levels. Plasma testosterone levels were related to the dose of injected TP, but were not influenced by photoperiod or pituitary transplants. Before the onset of TP injections, plasma LH and FSH levels in grafted and sham-grafted hamsters did not differ. In each of the three photoperiod conditions, injections of TP were consistently less effective in suppressing plasma gonadotropin levels in pituitary-grafted animals than in sham-grafted controls. These results indicate that PRL modulates the effects of exogenous testosterone on LH and FSH release in adult castrated male golden hamsters, this effect of PRL is due to reducing the sensitivity of the hypothalamic-pituitary system to feedback inhibition by testosterone, and suppression of pituitary PRL release in short photoperiod may be partially responsible for the concomitant increase in the sensitivity of LH and FSH release to inhibition by testosterone.
PMID: 6434293 [PubMed - indexed for MEDLINE]
1: Adv Exp Med Biol. 1987;219:153-75. Links
Role of prolactin in the regulation of sensitivity of the hypothalamic-pituitary system to steroid feedback.
Bartke A,
Matt KS,
Steger RW,
Clayton RN,
Chandrashekar V,
Smith MS.
Department of Physiology, Southern Illinois University, School of Medicine, Carbondale 62901.
During sexual maturation, pituitary gonadotropins stimulate the gonads to produce increasing amounts of biologically active steroids and yet gonadotropin release does not become suppressed until concentrations of sex hormones, LH and FSH, in peripheral circulation stabilizes at a higher adult level. There is a substantial amount of evidence that in many mammals, this transition from prepubertal to adult level of activity of the pituitary-gonadal axis is associated with a reduction in the sensitivity of the hypothalamic-adenohypophyseal system to negative feedback of gonadal steroids. In the female, these changes are accompanied by the appearance of positive estrogen feedback on gonadotropin release. In seasonal breeders, annual transitions between the periods of gonadal activity and quiescence are associated with corresponding shifts in the sensitivity to steroid feedback. Peripheral levels of pituitary prolactin (PRL) typically increase during sexual maturation and exhibit large seasonal fluctuations in response to changes in photoperiod and ambient temperature. We propose that PRL is one of the factors which regulate the sensitivity of gonadotropin release to gonadal steroid feedback. In hyperprolactinemic women, responsiveness to negative estrogen feedback increases, while LH response to positive estrogen feedback is reduced or absent. In hyperprolactinemic men, both LH and testosterone levels are reduced, implying increased sensitivity of LH release to negative testosterone feedback. In the male rat, both physiological amounts of PRL and experimentally-induced hyperprolactinemia increase the ability of exogenous testosterone to suppress LH and FSH release. Different regulatory mechanisms appear to operate in the seasonally breeding male golden hamster, in which short photoperiod causes concomitant suppression of PRL, LH, FSH and testosterone release. In this species, pharmacologic suppression of PRL release leads to increased responsiveness of plasma gonadotropin levels to negative feedback effects of testosterone, while PRL-secreting ectopic pituitary transplants exert an opposite effect. We have examined some of the suspected mechanisms of PRL modulation of testosterone feedback in male golden hamsters. In immature animals, the amount of cytoplasmic androgen receptors in the anterior pituitary was decreased by mild hyperprolactinemia and increased by treatment with bromocriptine, an inhibitor of PRL release. Bromocriptine increased pituitary androgen binding also in adult hamsters. These findings would imply that PRL modulates the responsiveness to negative steroid feedback at the pituitary level.(ABSTRACT TRUNCATED AT 400 WORDS)
PMID: 3324676 [PubMed - indexed for MEDLINE]
1: Endocrinology. 1983 Jan;112(1):22-8. Links
Increased sensitivity to the negative feedback effects of testosterone induced by hyperprolactinemia in the adult male rat.
McNeilly AS, Sharpe RM, Fraser HM.
High plasma levels of PRL induced by transplants of two donor pituitaries under the kidney capsule of adult male rats resulted in a prolonged suppression of plasma levels of LH and FSH although testosterone levels were maintained within normal limits. Castration of rats with pituitary transplants resulted in a normal though delayed rise in serum levels of both LH and FSH to levels equivalent to those in normal castrated controls. This increase in gonadotropin levels occurred in spite of maintenance of elevated PRL levels. Two experiments were carried out in which testosterone was restored after castration by Silastic testosterone-containing implants of various lengths (Exp 1:60, 30, and 10 mm; Exp 2: 30, 20, 10, 5, and 2 mm). In both experiments 60- and 30-mm testosterone implants prevented the postcastration rise in LH and FSH in both control and hyperprolactinemic rats. However, although the shorter testosterone implants delayed this rise in control rats, levels of LH and FSH increased by 4 days and were not significantly different from castrated rats without testosterone implants by 15 days after castration. In contrast, this rise in gonadotropins was abolished or considerably delayed by the shorter implants in hyperprolactinemic rats, demonstrating an increase in sensitivity of the hypothalamic pituitary axis to the negative feedback effects of testosterone in these animals. These results suggest that 1) to maintain suppression of gonadotropin secretion in hyperprolactinemia high levels of PRL alone are insufficient and gonadal steroids are required, and 2) high levels of PRL appear to sensitize the hypothalamic-pituitary axis to the negative feedback effects of gonadal steroids.
PMID: 6401176 [PubMed - indexed for MEDLINE]
1: J Endocrinol. 1999 Feb;160(2):197-203. Links
The antiprogestin RU486 dissociates LH and FSH secretion in male rats:
evidence for direct action at the pituitary level.
Sanchez-Criado JE, Bellido C, Tebar M, Ruiz A, Gonzalez D.
Department of Physiology, Faculty of Medicine, University of Cordoba, Spain.
Administration of 4 mg of the antisteroid RU486 over 8 consecutive days to adult male rats dissociated in vivo and in vitro gonadotrophin secretion, increasing FSH and decreasing LH secretion. In subsequent experiments we evaluated the involvement of testicular or adrenal secretory products, as well as hypothalamic LHRH, in the effects of 4 consecutive days of RU486 treatment on the secretion of gonadotrophins. The first day of RU486 injection was designated day 1, subsequent days being numbered consecutively. Groups of rats injected with oil (0.2 ml) or RU486 (4 mg) were: (i) injected s.c. from day 1 to day 4 with the antiandrogen flutamide (10 mg/kg); (ii) bilateral orchidectomized (ORCH) on day 1; and (iii) bilateral adrenalectomized (ADX) on day 1. Controls were given flutamide vehicle or were sham operated. To ascertain whether the secretion of LHRH is involved in the effects of RU486 on gonadotrophin secretion, we measured the LHRH secretion into the pituitary stalk blood vessels at 1100 h on day 5 in oil- or RU486-treated rats. Additional oil- and RU486-treated rats were injected i.p. with 100 ng LHRH at 1000 h on day 5, or s.c. with 1 mg LHRH antagonist (LHRH-ANT) at 1000 h on days 2 and 4. Controls were given saline. All animals were decapitated at 1100 h on day 5, trunk blood collected and serum stored frozen until FSH, LH and testosterone assays.%While ADX had no effect on FSH and LH secretion in either oil- or RU486-treated rats, the removal of androgen negative feedback with flutamide treatment or by ORCH substantially increased serum levels of FSH and LH in both oil- and RU486-treated rats, and thus annulled the effects of RU486. No differences in pituitary stalk plasma LHRH concentrations were found between oil- and RU486-treated rats. Injection of LHRH increased serum FSH and LH concentrations in oil-treated rats but only, and to a lesser extent, LH concentrations in RU486-treated rats. Treatment with LHRH-ANT decreased serum concentrations of FSH and LH in both oil- and RU486-treated rats. These results suggest that RU486 inhibited LHRH-stimulated LH secretion at the pituitary level, and that FSH secretion increased in response to a reduction in the negative feedback of androgen.
Effects of hyper- and hypoprolactinemia on gonadotropin secretion, rat testicular luteinizing hormone/human chorionic gonadotropin receptors and testosterone production by isolated Leydig cells.
Waeber C, Reymond O, Reymond M, Lemarchand-Beraud T.
The effect of prolactin (Prl) on gonadotropin secretion, testicular luteinizing hormone (LH)/human chorionic gonadotropin (hCG) receptors, and testosterone (T) production by isolated Leydig cells has been studied in 60-day-old rats treated for 4 days, 4 and 8 weeks with sulpiride (SLP), a dopaminergic antagonist, or for 4 days and 4 weeks with bromocriptine (CB), a dopaminergic agonist. Plasma Prl concentrations were significantly greater in the SLP groups (204 +/- 6 ng/ml) and lower in the CB groups (3.0 +/- 0.2 ng/ml) than those measured in the control groups (54 +/- 6 ng/ml). The plasma concentrations of gonadotropin were not affected by a 4-day treatment with SLP or CB, nor were they after a 4-week treatment with CB. However, the hyperprolactinemia induced by an 8-week treatment with SLP was associated with a reduced secretion of gonadotropin (LH, 16 +/- 4 vs. 35 +/- 6 ng/ml; FSH, 166 +/- 12 vs. 307 +/- 14 ng/ml). In SLP-induced hyperprolactinemia, a 30% increase in the density of the LH/hCG testicular binding sites was observed (178 +/- 12 fmol/mg protein), whereas a 60% decrease was measured in hypoprolactinemia (55 +/- 5 vs. control 133 +/- 5 fmol/mg protein). Plasma T levels were increased in 4-day and 4-week hyperprolactinemic animals (4.3 +/- 0.4 and 3.9 +/- 0.4 ng/ml, respectively), but returned to normal levels in the 8-week group (3.0 +/- 0.5 vs. C: 2.3 +/- 0.2 ng/ml). No T modifications were observed in hypoprolactinemic animals. Two distinct populations of Leydig cells (I and II) were obtained by centrifugation of dispersed testicular cells on a 0-45% continuous Metrizamide gradient. Both possess LH/hCG binding sites. However, the T production from Leydig cells of population II increased in the presence of hCG, whereas that of cell population I which also contain immature germinal cells did not respond. The basal and stimulated T secretions from cell populations I and II obtained from CB-treated animals were similar to controls, whereas from 4 days to 8 weeks of hyperprolactinemia, basal and hCG induced T productions from cell population II decreased progressively. These data show that hyperprolactinemia causes, in a time-dependent manner, a trophic effect on the density of LH/hCG testicular receptors; reduces basal and hCG-stimulated T production from isolated Leydig cells type II; and results in an elevated plasma T concentration which decreases with time. The latter suggests a slower T catabolism and/or an impaired peripheral conversion of T into 5 alpha-dihydrotestosterone (DHT). Although hypoprolactinemia is associated with a marked reduction in testicular LH receptors, it does not affect T production.
PMID: 6299412 [PubMed - indexed for MEDLINE]
Last edited:


 Please Scroll Down to See Forums Below
Please Scroll Down to See Forums Below 



.jpg)






.png)


 I just started taking it at .5mg E4D. Thanks
I just started taking it at .5mg E4D. Thanks